Best in Class Medical/Clinical Science Liaison Insights
Highlights of the report:
Download a PDF of these Highlights
Medical/clinical science liaisons represent an essential component of the manufacturer-payor relationship by acting as field-based clinical experts responsible for providing objective scientific education and support to address customers informational needs. HIRC's report, Best in Class Medical/Clinical Science Liaison Customer Insights, provides nominations of the best medical/clinical science liaisons, as well as the descriptive factors that operationally define the best-in-class. The report addresses the following questions:
- Which very large, large and mid-size firms' medical/clinical science liaisons receive the most best-in-class nominations?
- Which firms lead in best medical/clinical science liaison nominations across health plan pharmacy directors, health plan medical directors, and pharmacy benefit manager executives?
- Which medical/clinical science liaison attributes operationally define the best-in-class?
Key Finding: The most important factors driving nominations include: providing information (e.g., best practices, actionable market intelligence, clinical insights), responsiveness and follow-up, and product knowledge.
Medical/Clinical Science Liaisons from Novartis are Most Frequently Nominated as Best-in-Class. HIRC queried 65 commercial health plan and pharmacy benefit manager key decision-makers to learn which firms' medical/clinical science liaisons are best-in-class. Novartis leads with the most best-in-class nominations from managed markets customers, followed by AbbVie, Johnson & Johnson HCS, Lilly, Pfizer and Novo Nordisk.
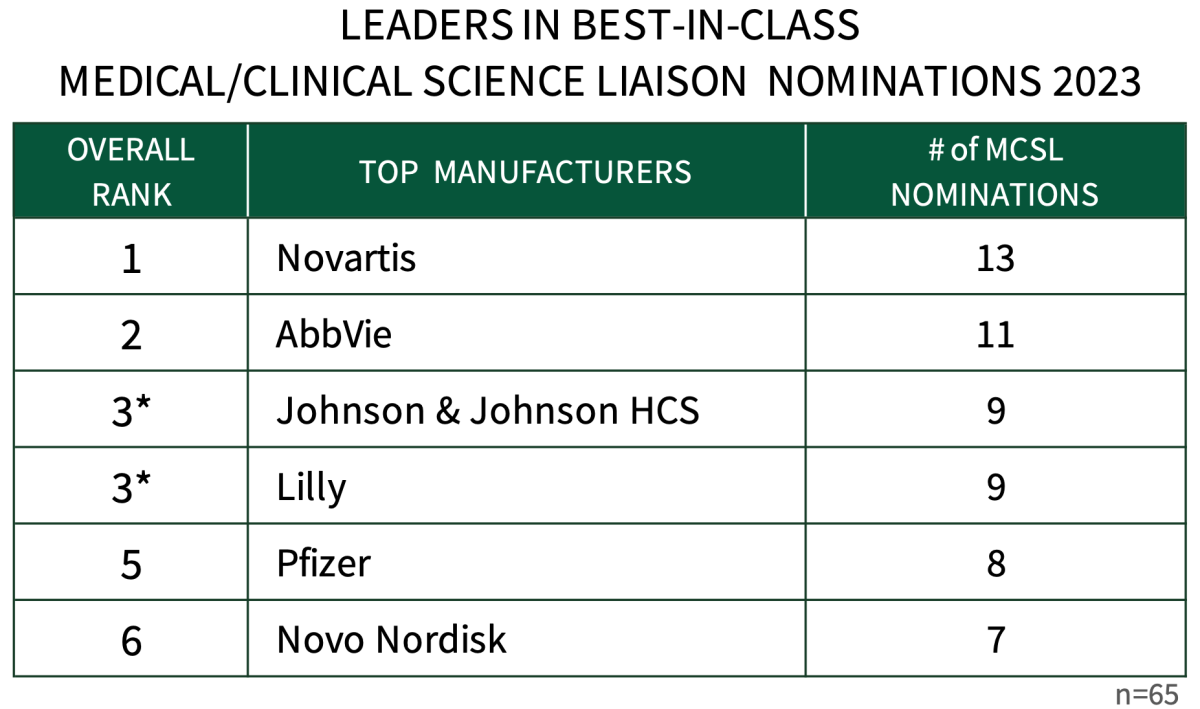
The full report provides a complete listing of best MCSL nominations as well as the rationale behind the nominations.
Five Factor Categories Operationally Define Best-in-Class Medical/Clinical Science Liaisons. Managed markets customers were asked to explain their rationale for best medical/clinical science liaison nominations. Analyses reveal that their evaluations fall within five key descriptive factor categories: (1) Knowledge, (2) Relationship Management, (3) Role Expectation, (4) General Impressions, and (5) Interpersonal & Social Skills.
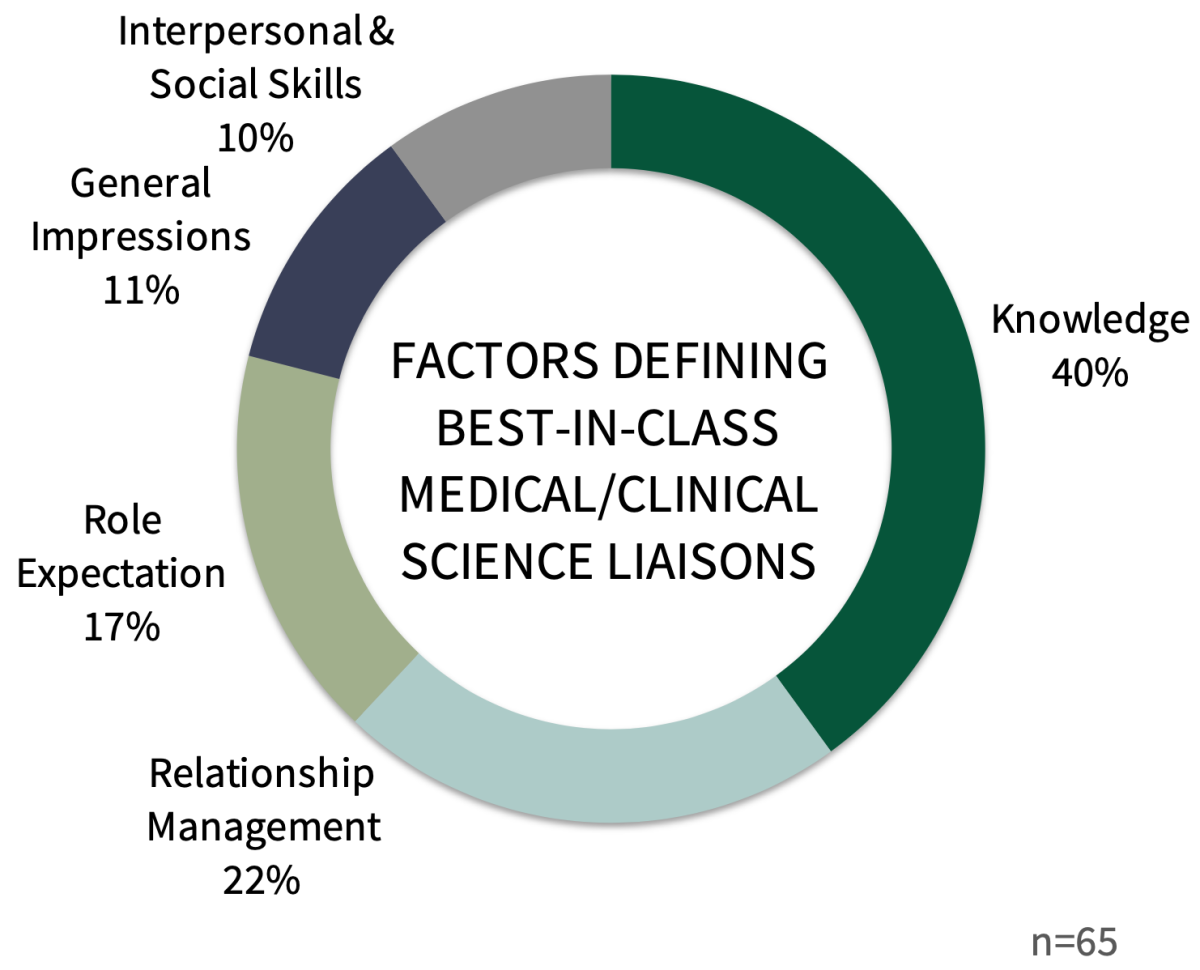
The best medical/clinical scientific liaisons in 2023 are often described by managed markets key-decision makers as (1) strong information providers of best practices, market intelligence, and clinical insights, (2) excellent in responsiveness and follow-up, and (3) demonstrating deep product knowledge.
Research Methodology and Report Availability. In April 2023, HIRC surveyed 65 key decision-makers from commercial health plans and pharmacy benefit managers. Online surveys and follow-up telephone interviews were used to gather information. The complete report, Best in Class Medical/Clinical Science Liaison Customer Insights, is available now to HIRC’s Best Programs and Best People subscribers at www.hirc.com.
Download a PDF of these Highlights
Download Full Report (Subscribers only) >


