Community Oncology Practices: Market Trends and Manufacturer Competitive Assessment
Highlights of the report:
Download a PDF of these Highlights
Community oncology practices (COPs) report a number of disruptive market trends in 2023 that directly impact their revenue and viability of current economic/business model. HIRC's report, Community Oncology Practices: Market Trends and Manufacturer Competitive Assessment, reviews COPs' needs, challenges, and concerns, top strategic imperatives for 2023, and provides an assessment of manufacturer engagement. The report addresses the following questions:
- What are COPs' top market concerns and strategic priorities in 2023?
- What is the status of COP activity related to oncology pharmacy and dispensing services (e.g., alternate site infusion, specialty pharmacy, etc.)?
- What is the status of oncology preferred drug lists, clinical pathways, and other utilization management tactics?
- Which firms are most often nominated as COPs' partner of choice? Which firms are nominated as providing the best oncology-related support offerings?
- How do pharmaceutical firms benchmark in account engagement and quality of oncology account managers, medical/clinical science liaisons, and field-based reimbursement managers?
Key Finding: Adequate staffing to support growth, broadening contracted payer base to drive patient volume, and developing cost-reduction initiatives are among oncology practices' top strategic priorities in 2023.
Top Market Trends for Community Oncology Practices in 2023. Community oncology practice respondents identify a number of market trends with the potential to impact their organizations, including (1) increasing complexity of the prior authorization process and administrative burdens from payers, (2) adopting oncology biosimilars, and (3) declining reimbursement from payers - both commercial and government.

The full report provides the complete listing of 50+ trends identified by oncology practices, as well as their top strategic priorities for the next 12-18 months.
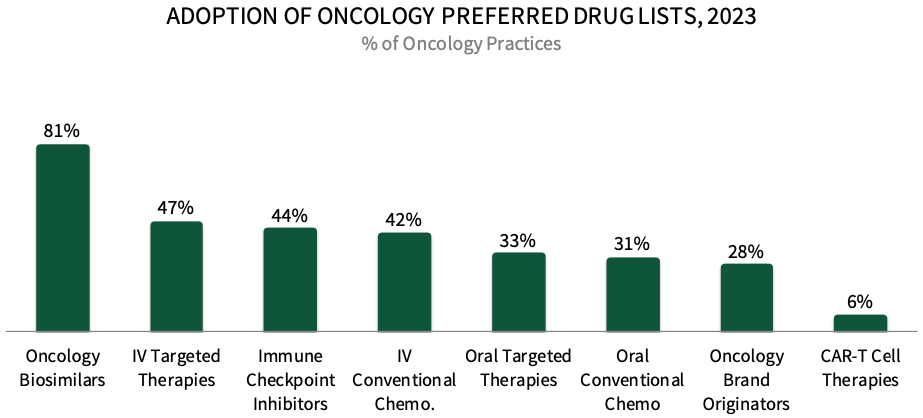
COPs Most Frequently Designate Preferred Products in Categories with Oncology Biosimilars. Panelists were asked to indicate whether their practice designates any oncology medications as preferred across eight medication types. About 81% of respondents in HIRC's sample report having preferred oncology biosimilar products in place in 2023, followed by 47% with preferred IV targeted therapies, and 44% with preferred immune checkpoint inhibitors. The full report examines biosimilars in detail, including which are preferred and how COPs promote their use.
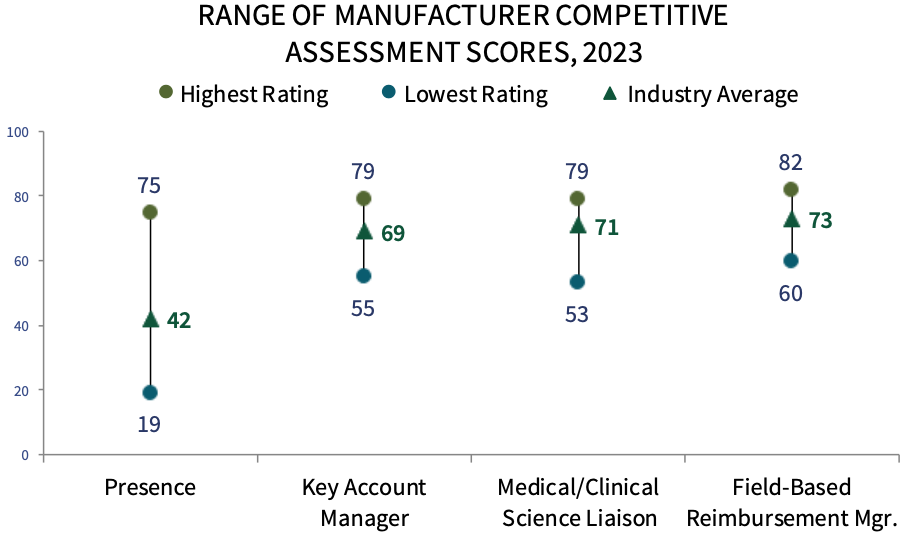
Pharmaceutical Manufacturer Competitive Assessment. COP respondents were asked to evaluate manufacturer customer-facing personnel, such as oncology account managers, medical/clinical science liaisons, and field-based reimbursement managers. Genentech, Pfizer Oncology, and Novartis rate highest in overall manufacturer field personnel engagement. The complete report provides COP executives' ratings of 26 firms active in the oncology space, as well as ratings of manufacturer's oncology-related support offerings and nominations for overall partner of choice.
Research Methodology and Report Availability. In January HIRC surveyed 36 executives from community oncology practices. Online surveys and follow-up telephone interviews were used to gather information. The full report, Community Oncology Practices: Market Trends and Manufacturer Competitive Assessment, is available now to HIRC’s Managed Oncology subscribers at www.hirc.com.
Download a PDF of these Highlights
Download Full Report (Subscribers only) >

