Integrated Delivery Networks: Evaluation of Pharmaceutical Manufacturer Programs and Support Offerings
Highlights of the report:
Download a PDF of these Highlights
Manufacturer-sponsored support programs and resources—when relevant and impactful—can spark stronger collaboration and drive strategic partnerships with Integrated Delivery Network (IDN) customers. HIRC's report, Integrated Delivery Networks: Evaluation of Pharmaceutical Manufacturer Programs and Support Offerings, provides IDN ratings of manufacturer resources, examines the most valuable programs being offered, and identifies opportunities for future support. The report addresses the following:
- Which pharmaceutical firms receive the greatest number of "most valuable" program/resource nominations across 11 priority therapeutic areas?
- What are some examples of the most valuable programs in key disease areas?
- How do 25+ manufacturers rate in presence and overall value of programs/resources?
- How often are IDNs interacting with manufacturers and their programs/resource offerings based on their role/responsibility type?
- How often are 25+ manufacturers providing support to their key accounts across eight unique program types?
- What program opportunities exist to better meet the current needs of IDN customers?
Key Finding: Leading pharmaceutical firms in IDN programs & resource engagement are often providing tangible solutions to real problems in patient care and/or support that directly ties to IDNs’ quality, population health, and cost management goals.
Pfizer, Novo Nordisk, and J&J Innovative Medicine Among Leaders in Most Valuable Resource Offerings for IDNs. IDN executives were asked to consider and nominate a manufacturer-sponsored program or resource offering that has provided the most value across eleven broad, high priority disease areas. Pfizer receives the greatest number of “most valuable” program/resource nominations, followed closely Novo Nordisk, J&J Innovative Medicine, and AbbVie. Nominations were most often provided for patient-centered resources supporting diabetes/obesity, oncology, airway diseases, and behavioral health. Pfizer in particular is noted for its commitment to improving population health through partnerships in key therapeutic areas.
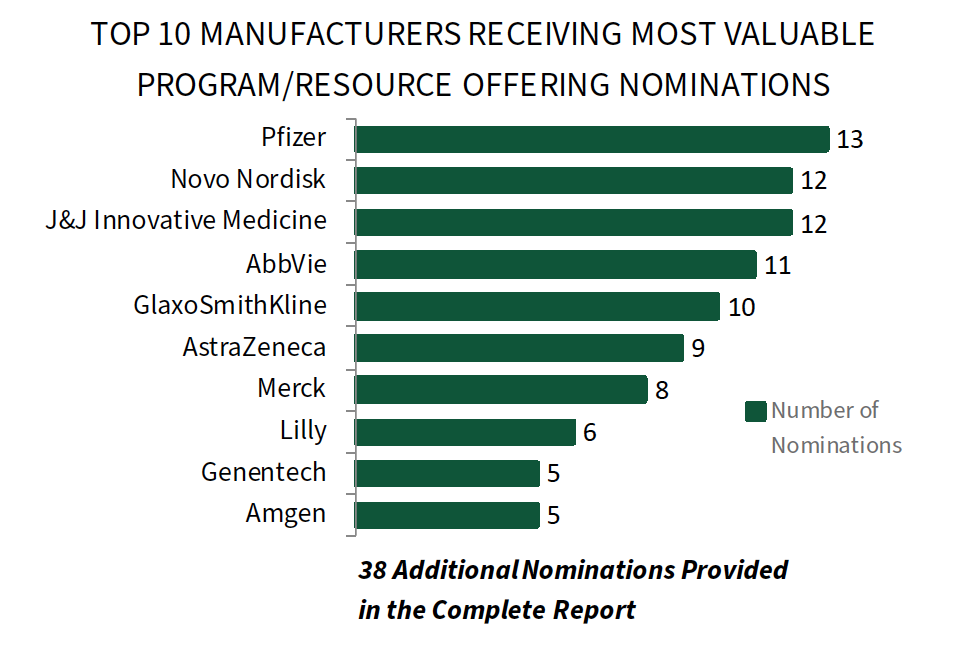
The complete report provides the full listing of 120 nominations received for 29 pharmaceutical firms across 11 disease states.
Benchmarking 25+ Manufacturers in IDN Programs/Resource Engagement. For a listing of 25+ companies active in IDN engagement, HIRC reviews the programs/resource support landscape across three key metrics: 1) Presence (IDN has had experience with a manufacturer's program/resource in the last 12-18 months, 2) IDN ratings of firm's Value of Programs & Resources, and 3) Program Engagement across 8 unique program types. A handful of manufacturers are consistently positioning resources and programs with their IDN target accounts across multiple program categories.
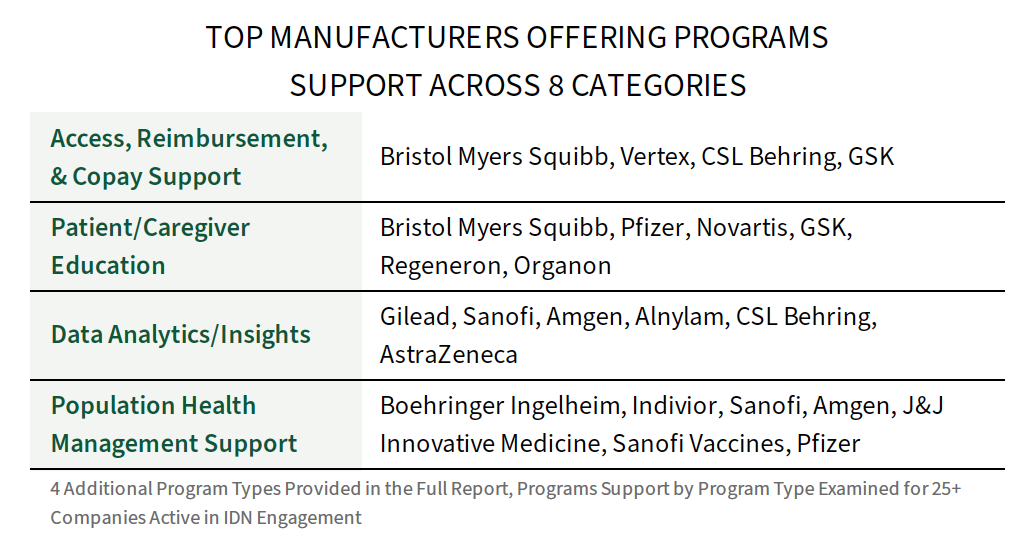
Program engagement benchmarks span the following 8 program types and 11 broad therapeutic areas:
Therapeutic Areas Covered:
- Airway Disease
- Behavioral Health
- Cardiovascular Disease
- Diabetes/Obesity
- Inflammation & Immunology
- Neurological Diseases
- Oncology/Cancer
- Ophthalmology
- Rare Disease
- Vaccines
- Women's Health
Program/Resource Types Covered:
- Access, Reimbursement, & Copay Support
- Clinical Staff Training/Education
- Data Analytics/Insights
- Digital Engagement/Telehealth Support
- Medication Adherence Support
- Patient/Caregiver Education
- Population Health Management Support
- Social Determinants of Health/Health Equity Support
Research Methodology and Report Availability. In July/August, HIRC surveyed 53 pharmacy directors, medical directors, and quality/population health personnel from IDNs ranging in size and geographic location. The full report, Integrated Delivery Networks: Evaluation of Pharmaceutical Manufacturer Programs and Support Offerings is part of the Organized Providers Service, and is now available to subscribers at www.hirc.com.
Download a PDF of these Highlights
Download Full Report (Subscribers only) >


